Finding the Distillation Boiling Point for a reflux still

To get the perfect distilled brew, it all revolves around the distillation boiling point. Today, we’re focusing on boiling points, particularly using a reflux still—a still that helps purify liquids.
Let’s break this down into simple terms and explore why boiling points are so crucial (vital) in the distillation process.
What is a Distillation Boiling Point?
Distillation is where liquids turn into vapour and then back into liquid to separate them based on one simple thing: the distillation boiling points. The boiling point is the temperature at which a liquid becomes a vapour.
This is important because, in a mixture of liquids, each type of liquid turns into vapour at its unique temperature (see below). The distillation process uses this trick to separate those liquids.
In a reflux still, the magic gets even better! It has a special part called a condenser that cools the vapour back into a liquid. This lets it catch the purest parts of the liquid by allowing the vapour to go up and down several times, making it cleaner each time.
What are the Distillation Boiling Points Temperatures – Foreshots, Heads, Hearts, Tails?
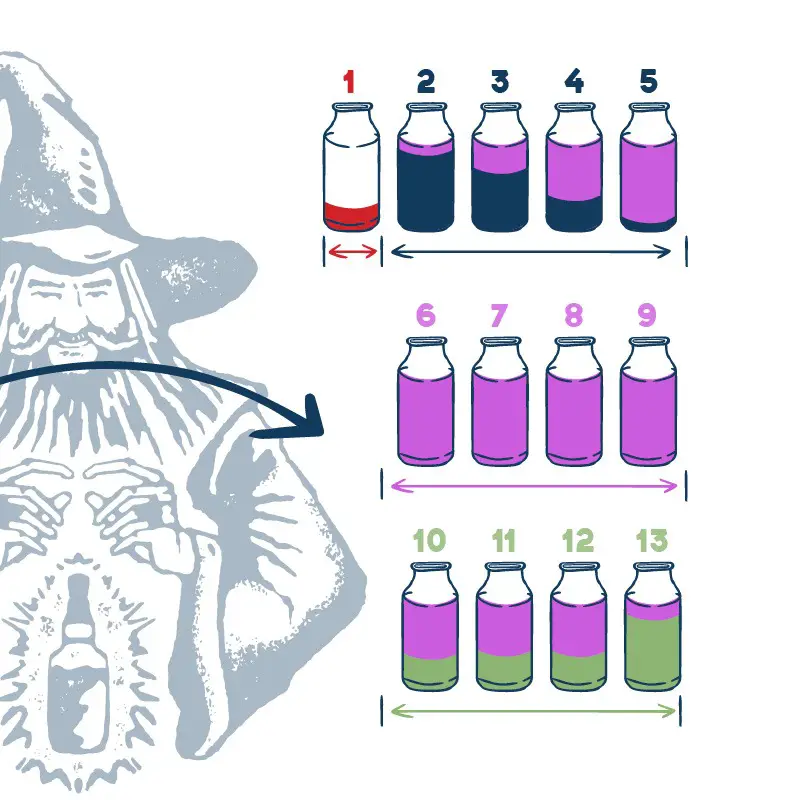
During the distillation method, not all parts of the mixture are equal. Different parts come out at different times, and they are called foreshots, heads, hearts, and tails.
Each part has its own boiling point range:
Foreshots And Heads – Temperature range 56 > 77.5°C (132.8 > 171.5°F) = Discard this alcohol
Hearts – Temperature range 78.3 > 81.5°C (172.9 > 178.7°F) = Collect this alcohol boiling range
Tails – Temperature ranges from 82°C (179.6°F) = Can be discarded or added to the next batch
The temperature guide is below:
| Acetone – Foreshots | 56.6°C or 133.8°F |
| Methanol- Foreshots | 64°C or 147.2°F |
| Ethyl Acetate – Heads | 77.1°C or 170.8°F |
| Ethanol Range – Hearts | 78.3 > 81.5°C or 172.9 > 178.7°F |
| 2 Proponal – Tails | 82°C or 179.6°F |
| 1 Proponal – Tails | 97°C or 206.6°F |
| Fuesel oils – Tails | 94 > 95°C or 201.2 > 203°F |
Why is the Boiling Point Important in a simple Distillation?
The boiling point in distillation is like a special code that tells us how to separate the parts of a mixture perfectly. Here’s why it’s so important:
Boiling points tell us exactly when to collect the good stuff and when to avoid the not-so-good parts.
This knowledge is key to making everything from perfumes to pharmaceuticals, and of course, all your favourite spirits!
What to know about the distillation process?
Learning about the different boiling points in distillation is like getting a sneak peek behind the curtain of a magic show. Here’s what to remember:
So, next time you enjoy a product made through distillation, it all starts with the distillation boiling points!
| Acetone – Foreshots | 56.6°C or 133.8°F |
| Methanol – Foreshots | 64°C or 147.2°F |
| Ethyl Acetate – Heads | 77.1°C or 170.8°F |
| Ethanol Range – Hearts | 78.3 > 81.5°C or 172.9 > 178.7°F |
| 2 Proponal – Tails | 82°C or 179.6°F |
| 1 Proponal – Tails | 97°C or 206.6°F |
| Fuesel oils – Tails | 94 > 95°C or 201.2 > 203°F |
Download our Distilling Cuts Chart here
Yes I want this epic PDF plus get tips and tricks! You will be joining our distilling mail list. 🙂
Last Updated on Nov 7, 2023 by The Brew Mechanic
Disclosure: I may receive affiliate compensation for some of the links below at no cost to you if you decide to purchase a product or service. You can read our affiliate disclosure in our privacy policy. The information provided is for entertainment only.

With 35 years of knowledge of being a chemical engineer in alcohol manufacturing plants, my mission is to teach the next generation of home distilling alcohol brewers at a supernatural speed.
My reviews are based on real-life experiences with reflux stills, sugar wash, troubleshooting and mystical chemical reactions.