Methanol In Homemade Alcohol (Wood Alcohol) while brewing

Disclaimer: This post might include affiliate links, through which I may earn a small commission without any extra cost to you. Additionally, I am an Amazon Associate and earn from eligible purchases. All the products and services I suggest are ones I have personally used or would use. Thank you very much for your support if you decide to buy through any of my links!
Come join the Distilling Squad!
Get the best fundamental tips & tricks here. Woohoo!
What is Methanol
Methanol in homemade alcohol is a colourless, flammable liquid with a very distinct smell and taste when pure. However, it is difficult to smell or taste when mixed with a soft drink like coke.
The danger here is that when concentrated to high levels, it can be fatal if consumed in large quantities, which ends up being methanol poisoning.
The body converts methanol to formaldehyde and formic acid, which can cause blindness and possibly lead to death.
Where can I find methanol?
Methanol, also known as methyl alcohol or wood alcohol, is a toxic alcohol that can be present in some alcoholic beverages:
Other sources of methanol include:
The Formulae of Methanol and Ethanol
Methanol Formulae – CH3OH
Ethanol Formulae – CH3CH2OH
As can be seen, there is a distinct difference in the mole weight and boiling point of ethanol versus methanol. So for you to get methanol into the ethanol should never happen!
How Is Methanol Removed From the Fermented Solution
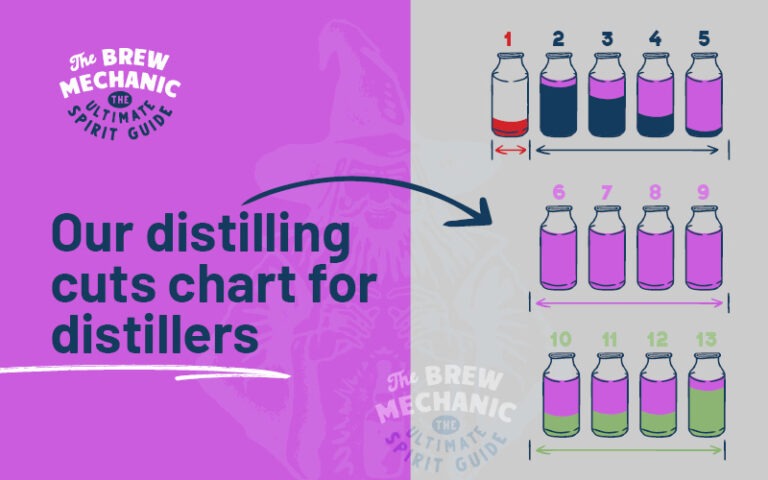
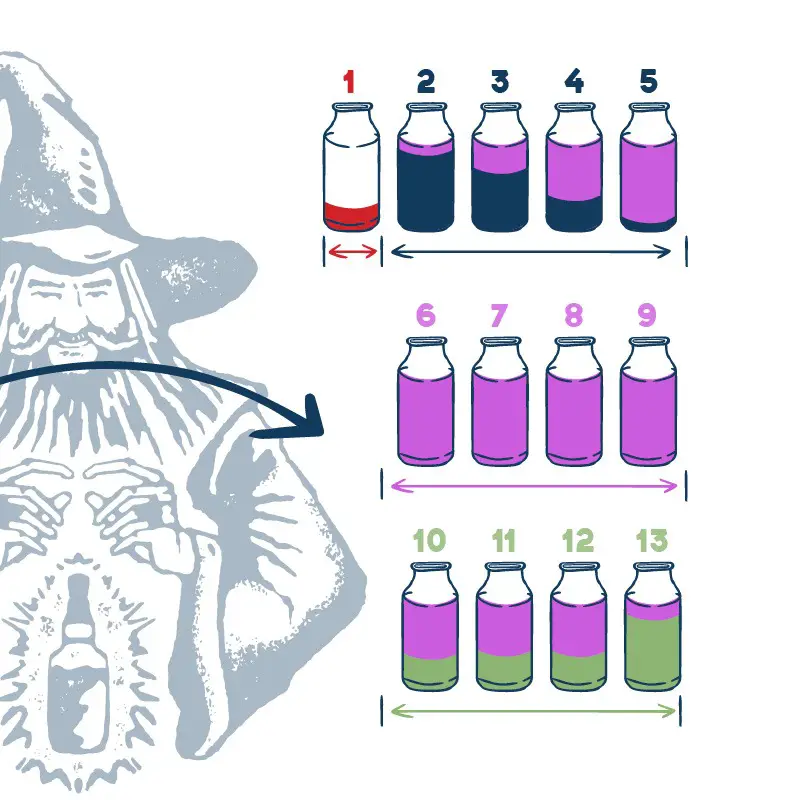
Distillation is used to separate the CH3 (primary alcohol) methanol in homemade alcohol from the mixture.
Note: Methanol BP is 64.7°C (148.4°F) and Ethanol BP is 78°C (172.4°F), the mole weights differ by up to 30 points. This will be removed as foreshots and heads with other undesirables when making cuts.
How Can We Get Methanol Into Spirits That We Drink?
Methanol must be removed during distillation of the spirits. For you to get methanol into spirits, the distiller has not separated the foreshots and heads from the hearts… That means bad quality and a bad distiller.
How Dangerous Is This?
How Can Methanol Be Tested?
Good to know – how can you sense methanol in your drink
Unfortunately, once you mix the drink it is almost impossible to tell (smell and taste) if it is contaminated. Methanol mixes with water like ethanol plus the methanol is colourless.
Should you get sick or have one hell of a hangover then you have drunk spirits that have not been correctly separated in the distillation phase. Buying from stalls and any dodgy bars.
From these stalls, you must smell the spirits before they dilute with a soda/cool drink. Should it not smell right (medical smell) or taste, colour is wrong don’t drink it.
Last Updated on Jan 09, 2025 by The Brew Mechanic
Disclosure: I may receive affiliate compensation for some of the links below at no cost to you if you decide to purchase a product or service. You can read our affiliate disclosure in our privacy policy. The information provided is for entertainment only.

With 35 years of knowledge of being a chemical engineer in alcohol manufacturing plants, my mission is to teach the next generation of home distilling alcohol brewers at a supernatural speed.
My reviews are based on real-life experiences with reflux stills, sugar wash, troubleshooting and mystical chemical reactions.