Methanol Boiling Point: Its Importance in Alcohol Distillation

Disclaimer: This post might include affiliate links, through which I may earn a small commission without any extra cost to you. Additionally, I am an Amazon Associate and earn from eligible purchases. All the products and services I suggest are ones I have personally used or would use. Thank you very much for your support if you decide to buy through any of my links!
Come join the Distilling Squad!
Get the best fundamental tips & tricks here. Woohoo!
Methanol, also known as methyl alcohol, is the nasty stuff that you get when distilling alcohol. Understanding the methanol boiling point (64.7°C) is crucial for various professionals, from chemical manufacturers to fuel producers, and especially for us distillers of alcoholic beverages.
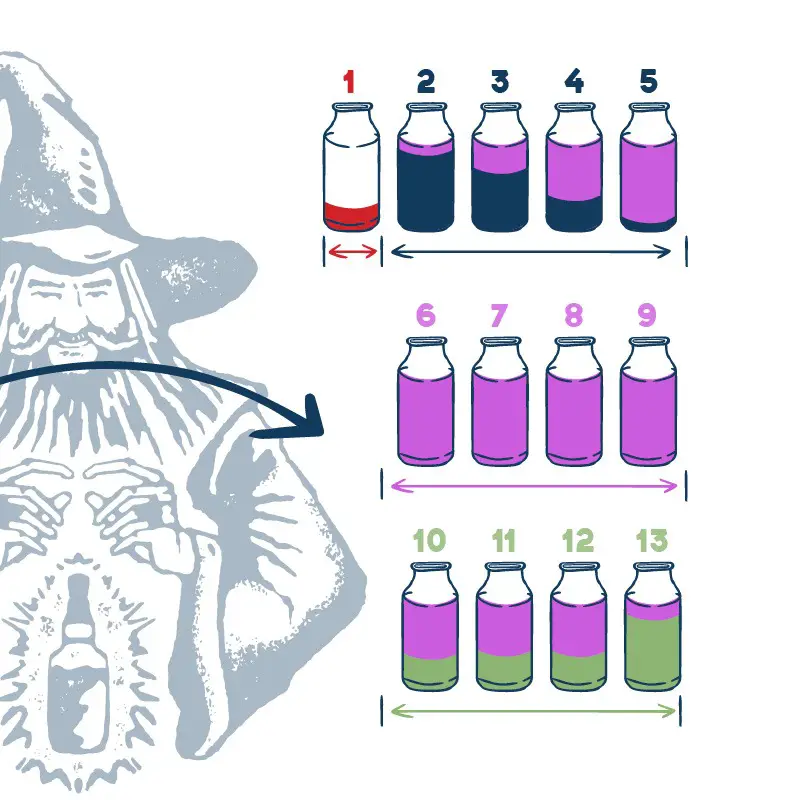
We have all heard of methanol poisoning which is when methanol mixes into the hearts cut. It can cause blindness and also high consumption can possibly lead to death.
Basically, we want the methanol and ethanol to be separate. Period!
We explain the reason for the methanol boiling point 64.7°C (148.5°F) and the importance of separating it during the cuts.
The methanol boiling point basics
At standard pressure, pure methanol boils at 64.7°C (148.5°F). This means, that when heated to this temperature, methanol transitions from a liquid to a gas.
Boiling points can change based on pressure and the presence of other substances. But the difference is so subtle that you need to make sure you boil off the methanol into the foreshots and heads then toss it. No questions asked.
Why Does Methanol’s Boiling Point Matter in Distillation?
We know Methanol, also called wood alcohol, is a toxic substance. While small amounts might not induce immediate harm like orange juice.
But exposure to methanol and ingesting larger quantities can lead to blindness, organ damage, or even death.
That is why we HIGHLY recommend starting with the sugar wash and reflux distilling first. As there is no chance of getting methanol is this method as it CAN NOT be chemically produced due to no pectin.
The cuts temperature chart is below:
| Acetone – Foreshots | 56.6°C or 133.8°F |
| Methanol – Foreshots | 64°C or 147.2°F |
| Ethyl Acetate – Heads | 77.1°C or 170.8°F |
| Ethanol Range – Hearts | 78.3 > 81.5°C or 172.9 > 178.7°F |
| 2 Proponal – Tails | 82°C or 179.6°F |
| 1 Proponal – Tails | 97°C or 206.6°F |
| Fuesel oils – Tails | 94 > 95°C or 201.2 > 203°F |
Download our Distilling Cuts Chart here
Yes I want this epic PDF plus get tips and tricks! You will be joining our distilling mail list. 🙂
Methanol and ethanol In spirit production:
Like with anything, you need to understand the different boiling points in the distillation process to get our desired alcohol! Woohoo.
The cuts chart is here again for you to understand.
| Acetone – Foreshots | 56.6°C or 133.8°F |
| Methanol – Foreshots | 64°C or 147.2°F |
| Ethyl Acetate – Heads | 77.1°C or 170.8°F |
| Ethanol Range – Hearts | 78.3 > 81.5°C or 172.9 > 178.7°F |
| 2 Proponal – Tails | 82°C or 179.6°F |
| 1 Proponal – Tails | 97°C or 206.6°F |
| Fuesel oils – Tails | 94 > 95°C or 201.2 > 203°F |
The Key points about the properties of methanol:
The methanol boiling point plays a critical role in distillation:
Remember: The methanol boiling point of 64.7°C (148.5°F) is a critical parameter for both the safety and quality of distilled products and industrial processes.
Last Updated on Feb 15, 2024 by The Brew Mechanic
Disclosure: I may receive affiliate compensation for some of the links below at no cost to you if you decide to purchase a product or service. You can read our affiliate disclosure in our privacy policy. The information provided is for entertainment only.

With 35 years of knowledge of being a chemical engineer in alcohol manufacturing plants, my mission is to teach the next generation of home distilling alcohol brewers at a supernatural speed.
My reviews are based on real-life experiences with reflux stills, sugar wash, troubleshooting and mystical chemical reactions.